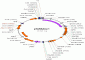
Cycle
Sequence Reactions For Large Insert
Plasmid Templates |
 |

The following dye-labeled
terminator reaction chemistries have been designed to balance
conservation of reagents with the resulting sequence product
signal strength. When coupled with BACPAC Resource template
preparation, N-free read lengths of >400nts can be expected.
More emphasis can be placed on reagent conservation by further
volume reduction or dilution; or on sequence product signal
strength by increasing the reaction size.
Cycle Sequence
Chemistry Conditions:
| Chemistry3 |
Reaction Size4 |
Final Vol (ul) |
Primer Vol (10pmol/ul) |
Reaction Mix vol
(ul) |
Template vol (ul) |
| ABI dRhod term |
1.25x |
25 |
1 |
10 |
14 |
| ABI BD term |
0.75x |
15 |
1 |
6 |
8 |
CycleSequenceConditions:
1) Initial denaturation step: 96oC 4min.
2) denaturation: 96oC 10sec.
3) annealing: 50oC 10sec.
4) extension: 60oC 4min. run steps 2-4 for 100 cycles.
5) hold: 4-10oC.
Primers Useful For BAC and PAC Vectors:
T7.29: 5’ gccgctaatacgactcactatagggagag 29mer
T7: 5’ taatacgactcactataggg 20mer
gSP6: 5’ gttttttgcgatctgccgtttc 22mer
Carlo Artieri (Simon
Frasier University) notified us per email (January 6, 2005)
that the standard BAC-end sequencing (BES)primer from the
SP6 end (as recommended on our web site)did not work well
with the pTARBAC2.1 vector (in the salmon BAC library, CHORI-214).
A new primer designed at Simon Frazier University worked
flawlessly to obtain BES data for more than 4,300 BAC clones.
The new primer is closer to the vector/insert junction and
has the following sequence: actgtggcttgttttacaattt. (Personal
Communication).
3Stock
ABI Ready Reaction mixes are supplemented with 4mM MgCl2
4Refers to ABI 1x (20ul) reaction
5Annealing temp can be adjusted, based on Tm of
primers used
Academic and commercial
users should contact Pieter J. de Jong ([email protected],
fax: (510) 450-7924). For hybridization membranes, and
individual clones, please contact BACPAC Resources ([email protected]).
|

